Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Natural Synthesis, Characterisation, and Photocatalytic Performance of Nanoparticles made of Manganese Dioxide using Manganese Acetate Tetrahydrate as Precursor
Authors: Anil Kumar, Dr. Anil Kumar Shrotriya
DOI Link: https://doi.org/10.22214/ijraset.2024.63874
Certificate: View Certificate
Abstract
This study describes a straightforward, effective, and environmentally benign method for producing manganese dioxide nanoparticles (MnO2 NPs) using Nyctanthes arbor-tristis leaf extract. It was discovered by Fourier-transform infrared spectra that the plant extract contributed to the creation of MnO2 NPs. The band gap of the synthesised MnO2 NPs was identified as the source of the absorption peaks observed in the UV–visible absorption spectra at 412 nm. X-ray diffraction analysis was used to identify the crystal phase of the MnO2 NPs and validate the development of crystalline MnO2 NPs. According to the X-ray diffraction pattern, MnO2 NPs have crystalline nature. Furthermore, the synthesised MnO2 NPs\' was revealed by field emission scanning electron microscopy investigation with EDS which gives Information about Compositional elements. MnO2 NPs have the ability to degrade MB dyes in the UV-Visible light spectrum by photocatalysis. Using Methylene Blue as an organic pollutant, the photocatalytic activities for the dye degradation of MnO2 NPs were assessed.
Introduction
I. INTRODUCTION
Nanomaterials have emerged as unique antibacterial agents with unique physicochemical features and a high surface area to volume ratio (Gatoo, Naseem, Arfat, Dar, Qasim, & Zubair, 2014). Additionally, nanomaterials are being used in medicine to find and deliver therapeutic drugs for a variety of illnesses (Bhatia, 2016). In comparison to other materials, metal nanoparticles (NPs) have superior surface area to volume ratio, surface energy, spatial confinement, and decreased imperfections. These properties make them reliable in physicochemical, electrical, mechanical, magnetic, thermal, optical, and biological processes (Joudeh & Linke, 2022). The wide range of uses for manganese oxide nanoparticles (NPs) in several sectors, including microelectronics (Xia, Wan, Yan, & Lu, 2014), chemical sensing devices (Dawadi, Gupta, Khatri, Budhathoki, Lamichhane, & parajuli, 2020), optoelectronics, rechargeable batteries, ion-sieves and catalysis (Debnath, Roy, Kapri, & Bhattacharyya, 2016), has drawn attention. Mn is the twelfth most abundant element on Earth and the third most common transition element after Fe and Ti (Hoseinpour, Souri, & Ghaemi, 2018). Moreover, because of the availability of several attachments for a variety of uses, they are based against earth additional elements. Additionally, the photocatalytic activity of manganese dioxide nanoparticles (MnO2 NPs) for dye degradation is good. The most popular methods for nanoscale synthesis include electrochemical and photochemical reduction techniques, chemical precipitation, sol-gel, solvothermal/hydrothermal, solid-state synthesis, and basic solution-based methods (Ding, Zheng, Ma, & Lin, 2020). Green fabrication is a preferable option since it is more environmentally friendly, even though physical and chemical processes can be used for nanoscale synthesis (Parveen, Banse, & Ledwani, Green Synthesis of Nanoparticles: Their Advantages and Disadvantages, 2016). Plant extracts can be used to manufacture nanoscales, which has advantages over other biological processes including the microbial method and the drawn-out process of maintaining cell cultures (jeevanandam, et al., 2022). The creation of metal and metal-oxide nanoscales is attributed to the antioxidant properties of plants. The use of extracts from numerous other herbs, including Xanthium strumarium, Euphorbia hirta, Azadirachta indica, Corriandrum sativum, Polyalthia longifolia,Camellia sinensis and Nelumbo nucifera, complies with the environmentally friendly concepts of green chemistry (Hoseinpour, Souri, & Ghaemi, 2018). This benignant reaction can be quickly and readily scaled up at room temperature and pressure. It can also be completed with ease.
As previously mentioned, utilising plant extracts for NP synthesis is referred to as a "green method." Numerous investigations, such as the synthesis of silver nanoparticles (NPs) utilising the catalytic activity of Malva parviflora leaf extract (Zayed, Eisa, & Shabaka, 2012), the synthesis of gold NPs using Phoenix dactylifera L. leaf extract (Zayed & Eisa, Phoenix dactylifera L. leaf extract phytosynthesized gold nanoparticles; controlled synthesis and catalytic activity, 2014), the biosynthesis of silver NPs using Ziziphus spina-christi, etc., all support this claim. This investigation used the green approach to generate MnO2 NPs, which were then characterised using Fourier-transform infrared (FTIR) analytical techniques, field emission scanning electron microscopy (FESEM),X-ray diffraction (XRD), ultraviolet-visible spectroscopy (UV-vis), synthesised MnO2 nanoparticles show photocatalytic activity for the breakdown of dye. The degradation of Methylene Blue dye was used to test the photocatalytic activity for dye degradation of the synthesised MnO2 NPs.
II. EXPERIMENTAL
A. Reagents
Manganese acetate tetra hydrate was bought from Fisher Scientific. methylene blue was purchased from Sigma Aldrich. DI water was taken from Organo biotech private limited. Leaves of Nyctanthes arbor-tristis plant were collected from a local garden. Whatman filter paper grade no. 1 size 125 mm was purchased from local vendor.
B. Instruments
Fourier Transform Infrared Spectroscopy (FTIR) was performed using the Shimadzu IRAffinity-1S model in Attenuated Total Reflectance (ATR) mode, covering a spectral range of 4000-400 cm-1. The FTIR analysis was conducted at a resolution of 8 cm-1 with a scanning speed of 64 scans/min. UV-Visible (UV-Vis) Spectroscopy for analyzing nanoparticle synthesis was recorded using the Shimadzu UV 1900i model. The particle size distribution of the nanoparticles was determined via Dynamic Light Scattering (DLS) spectroscopy, employing the Litesizer 500 model from Anton Paar. The morphological patterns and elemental composition of the synthesized samples were assessed using a Field Emission Scanning Electron Microscope (FESEM), operated at an energy setting of 10 KeV (Zeiss Supra 55VP, EDAX Ametek). Prior to imaging, the samples were mounted on carbon adhesive tape affixed to an aluminum stage and sputter-coated with gold using an Emitech K550X sputter coater from Labtech International for 120 seconds. The size and morphology of the synthesized nanoparticles were further characterized using Transmission Electron Microscopy (TEM) with a Jeol Jem-1400 model. A solution containing manganese dioxide nanoparticles was deposited onto a carbon-backed copper grid, which was then dried overnight at 40°C. The crystalline nature of the synthesized nanoparticles was determined by X-Ray Diffraction (XRD) using a Rigaku Ultima IV model, scanning from 5 to 60 degrees at a rate of 2 degrees/min with a CuKα radiation source.
C. Green Synthesis and Characterisation of MnO2 Nanoparticle using Manganese acetate tetra Hydrate as Precursor
- Preparation of Leaf Extract
Leaf extract from Nyctanthes arbor-tristis (commonly known as night-flowering jasmine) was prepared for the synthesis of nanoparticles. Fresh leaves of N. arbor-tristis were collected, thoroughly washed with deionized water to remove any surface contaminants, and then air-dried. Subsequently, 20 grams of the clean leaves were cut into small pieces and immersed in 100 milliliters of deionized water. This mixture was heated to 60°C and maintained at this temperature for 1 hour, during which the solution gradually turned a light green color, indicating the release of phytochemicals into the aqueous medium. The resultant leaf extract was then filtered through Whatman No. 1 filter paper to remove any solid plant residues, and the filtrate was collected for subsequent nanoparticle synthesis.
2. Synthesis of MnO2 Nanoparticles
Three sets of manganese dioxide MnO2 nanoparticles were synthesized using manganese acetate tetrahydrate Mn(CH3CO2)2.4H2O as the manganese precursor. Initially, 0.1 M manganese acetate was prepared by dissolving the appropriate amount in 10 mL of deionized water. To this solution, 1 mL, 3 mL, and 6 mL of the previously prepared Nyctanthes arbor-tristis leaf extract were added dropwise under continuous magnetic stirring.
The reaction mixtures were maintained at a constant temperature of 60°C and stirred continuously for 24 hours. During this process, the color of the solution changed from colorless to light yellow, indicating the formation of MnO2 nanoparticles. The resultant nanoparticle suspensions were then collected for further characterization.
III. RESULTS AND DISCUSSIONS
A. Fourier Transform Infrared Spectroscopy
The formation of MnO2 nanoparticles were confirmed by FTIR in ATR mode. The leaf extract and the salt precursor were also characterized via FTIR. The Figure 1. depicted the FTIR spectra of MnO2 nanoparticles. The leaf extract spectra showed band at ~3300 cm-1 arising from OH stretching vibrations due to the presence of various different types of flavonoids in the leaf extract. The salt precursor also showed bands at ~3300 cm-1 and ~3000 cm-1 due to the presence of OH and methyl groups respectively. The salt precursor showed bands at ~1552 cm-1 and ~1400 cm-1 due to CO asymmetric and symmetric stretching respectively. The ~1028 cm-1 band in salt corresponded to CH3 bending vibrations (Pang, Wu, Zhang, & Zhang, 2015). However, two new bands at ~1163 cm-1 and 829 cm-1appeared in the spectra of MnO2 nanoparticles. The appearance of these bands confirmed the formation of nanoparticles. However, the other peaks of salt at ~3300 cm-1, ~3000 cm-1, ~1552 cm-1, ~1400 cm-1, and ~1028 cm-1got slightly shifted in the MnO2-A1, MnO2-A2, and MnO2-A3 samples due to the formation of nanoparticles.
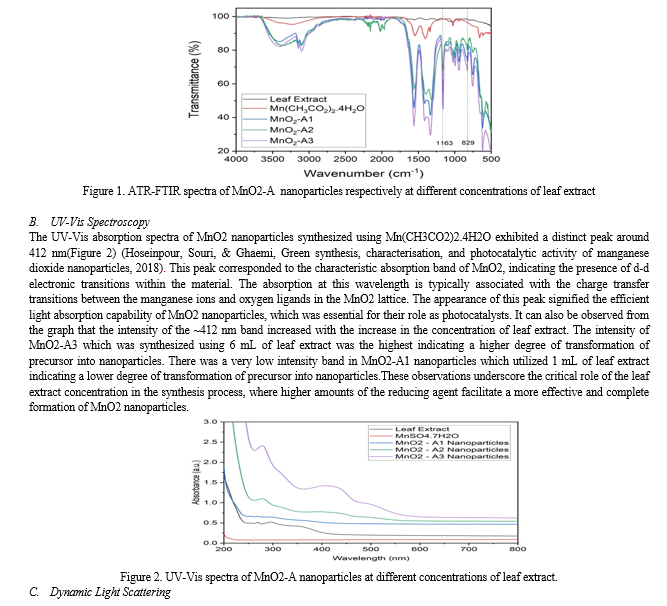
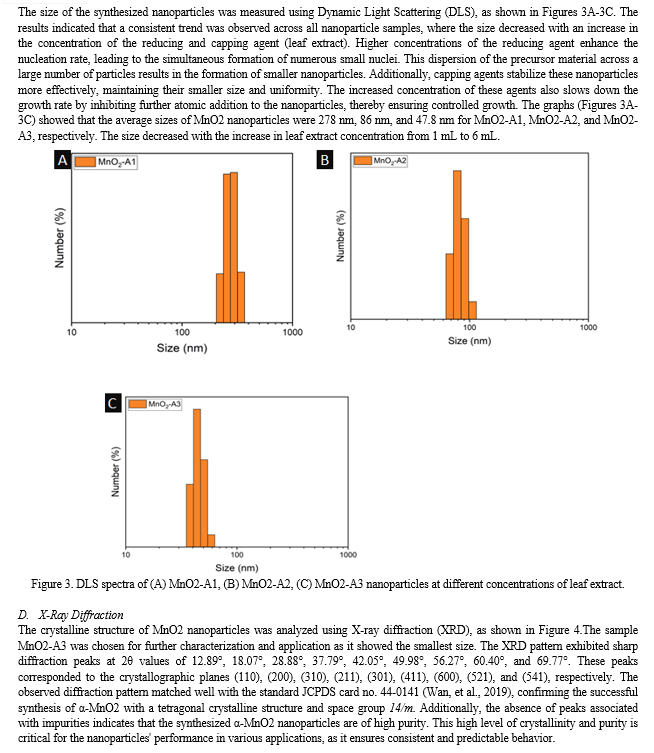
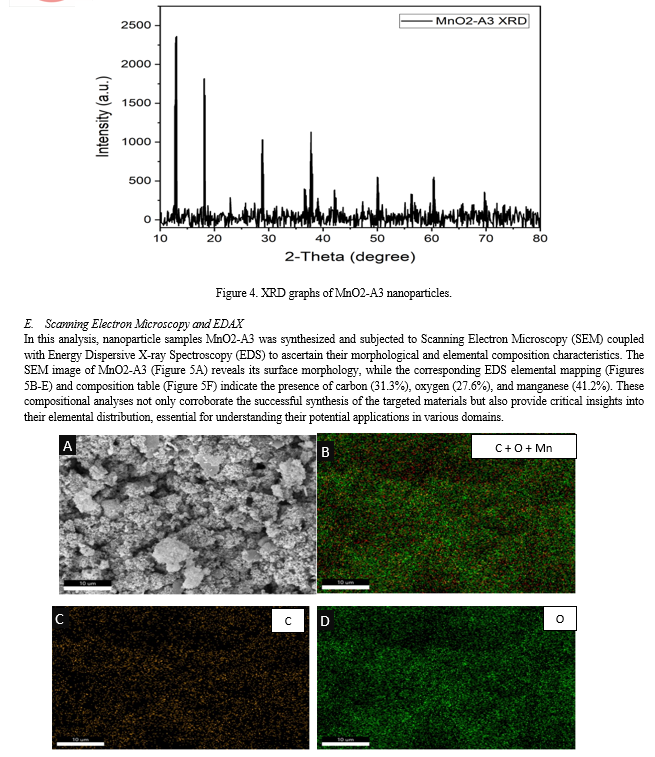
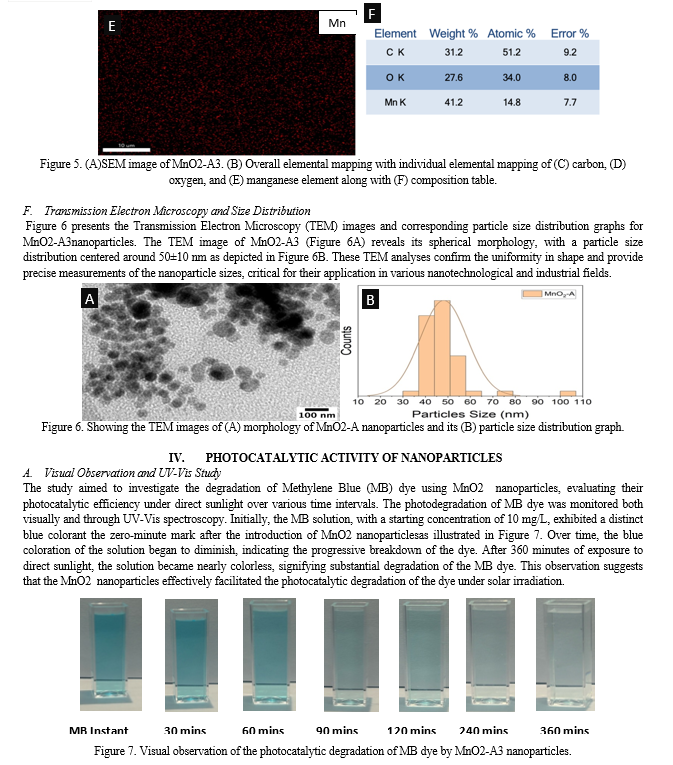
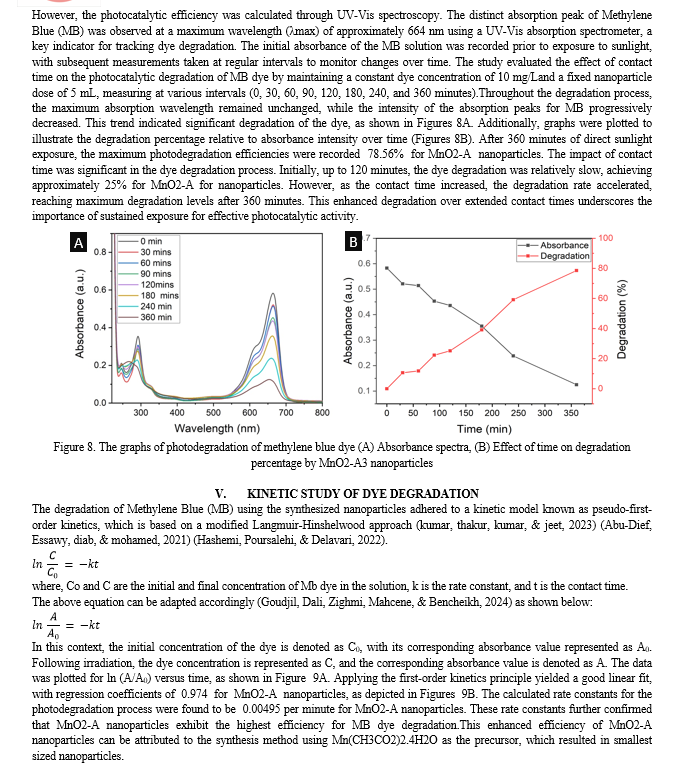
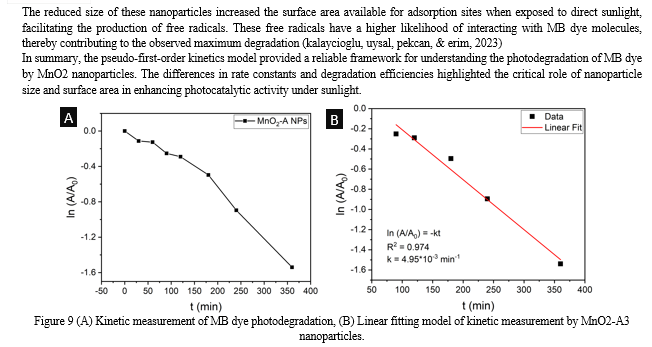
Conclusion
The synthesis and characterization of MnO2 nanoparticles using Nyctanthesarbor-tristis leaf extract have demonstrated the potential for green chemistry approaches in nanomaterial production. The comprehensive analysis of the synthesized nanoparticles revealed significant insights into their structural, morphological, and functional properties. The UV-Vis spectroscopy results demonstrated the efficient formation of MnO2 nanoparticles, with distinct absorption peaks at 412 nm, indicating their potential photocatalytic properties. The Dynamic Light Scattering (DLS) analysis confirmed that the size of the nanoparticles decreased with an increase in the concentration of the leaf extract, emphasizing the crucial role of the reducing and capping agent in controlling nanoparticle dimensions. X-ray diffraction (XRD) patterns confirmed the crystalline structures of MnO2 nanoparticles, demonstrating high purity and crystallinity, which are essential for their performance in various applications. Fourier Transform Infrared Spectroscopy (FTIR) further validated the formation of nanoparticles, with the appearance of new bands and shifts in existing bands indicating successful synthesis. Scanning Electron Microscopy (SEM) coupled with Energy Dispersive X-ray Spectroscopy (EDS) provided detailed morphological and elemental composition analysis, confirming the presence of key elements and supporting the successful synthesis of the targeted nanoparticles. Transmission Electron Microscopy (TEM) images and particle size distribution analyses revealed uniformity in shape and precise measurements of nanoparticle sizes, essential for their application in nanotechnology and industry. The synthesis process employed in this study offered significant advantages over conventional methods, primarily due to its eco-friendliness and sustainability. By utilizing Nyctanthesarbor-tristis leaf extract as a reducing and capping agent, the need for hazardous chemicals was eliminated, making the process safer and more environmentally benign. This approach aligned with the principles of green chemistry, promoting the use of renewable resources and reducing waste generation. The photocatalytic efficiency of the nanoparticles was evaluated through the degradation of Methylene Blue (MB) dye under direct sunlight. The UV-Vis spectroscopy data showed a significant decrease in the intensity of the characteristic absorption peak of MB, indicating substantial degradation of the dye. The study revealed that the photocatalytic efficiency with MnO2 (synthesized using Mn(CH3CO2)2.4H2O) achieving a 78.56% degradation rate after 360 minutes of sunlight exposure. The kinetic study of dye degradation followed pseudo-first-order kinetics, with calculated rate constants further confirming the superior photocatalytic activity of the composite. The comprehensive analysis underscored the influence of synthesis conditions, particularly the concentration of the leaf extract, on the structural and functional properties of the nanoparticles. The findings highlighted the potential of these biogenically synthesized MnO2 nanoparticles for efficient photocatalytic applications, emphasizing the critical role of nanoparticle size, surface area, and purity in enhancing their performance.
References
[1] Abu-Dief, A. M., Essawy, A. A., Diab, A. K., & Mohamed, W. S. (2021). Facile synthesis and characterization of novel Gd2O3–CdO binary mixed oxide nanocomposites of highly photocatalytic activity for wastewater remediation under solar illumination. Journal of Physics and Chemistry of Solids, 148, 109666. [2] Bhatia, S., & Bhatia, S. (2016). Nanoparticles types, classification, characterization, fabrication methods and drug delivery applications. Natural polymer drug delivery systems: Nanoparticles, plants, and algae, 33-93. [3] Dawadi, S., Gupta, A., Khatri, M., Budhathoki, B., Lamichhane, G., & Parajuli, N. (2020). Manganese dioxide nanoparticles: synthesis, application and challenges. Bulletin of Materials Science, 43, 1-10. [4] Debnath, B., Roy, A. S., Kapri, S., & Bhattacharyya, S. (2016). Efficient dye degradation catalyzed by manganese oxide nanoparticles and the role of cation valence. ChemistrySelect, 1(14), 4265-4273. [5] Ding, B., Zheng, P., Ma, P. A., & Lin, J. (2020). Manganese oxide nanomaterials: synthesis, properties, and theranostic applications. Advanced Materials, 32(10), 1905823. [6] Gatoo, M. A., Naseem, S., Arfat, M. Y., Mahmood Dar, A., Qasim, K., & Zubair, S. (2014). Physicochemical properties of nanomaterials: implication in associated toxic manifestations. BioMed research international, 2014(1), 498420. [7] Goudjil, M. B., Dali, H., Zighmi, S., Mahcene, Z., & Bencheikh, S. E. (2024). Photocatalytic degradation of methylene blue dye with biosynthesized Hematite ?-Fe2O3 nanoparticles under UV-Irradiation. Desalination and Water Treatment, 317, 100079. [8] Hashemi, E., Poursalehi, R., & Delavari, H. (2022). Structural, optical and photocatalytic activity of multi-heterojunction Bi2O3/Bi2O2CO3/(BiO) 4CO3 (OH) 2 nanoflakes synthesized via submerged DC electrical discharge in urea solution. Nanoscale Research Letters, 17(1), 75. [9] Hoseinpour, V., Souri, M., & Ghaemi, N. (2018). Green synthesis, characterisation, and photocatalytic activity of manganese dioxide nanoparticles. Micro & Nano Letters, 13(11), 1560-1563. [10] Jeevanandam, J., Kiew, S. F., Boakye-Ansah, S., Lau, S. Y., Barhoum, A., Danquah, M. K., & Rodrigues, J. (2022). Green approaches for the synthesis of metal and metal oxide nanoparticles using microbial and plant extracts. Nanoscale, 14(7), 2534-2571. [11] Joudeh, N., & Linke, D. (2022). Nanoparticle classification, physicochemical properties, characterization, and applications: a comprehensive review for biologists. Journal of Nanobiotechnology, 20(1), 262. [12] Kalayc?og?lu, Z., O?zug?ur Uysal, B., Pekcan, O., & Erim, F. B. (2023). Efficient photocatalytic degradation of methylene blue dye from aqueous solution with cerium oxide nanoparticles and graphene oxide-doped polyacrylamide. ACS omega, 8(14), 13004-13015. [13] Pang, S. F., Wu, C. Q., Zhang, Q. N., & Zhang, Y. H. (2015). The structural evolution of magnesium acetate complex in aerosols by FTIR–ATR spectra. Journal of Molecular Structure, 1087, 46-50. [14] Parveen, K., Banse, V., & Ledwani, L. (2016, April). Green synthesis of nanoparticles: Their advantages and disadvantages. In AIP conference proceedings (Vol. 1724, No. 1). AIP Publishing. [15] Wan, X., Yang, S., Cai, Z., He, Q., Ye, Y., Xia, Y., ... & Liu, J. (2019). Facile synthesis of MnO2 nanoflowers/N-doped reduced graphene oxide composite and its application for simultaneous determination of dopamine and uric acid. Nanomaterials, 9(6), 847. [16] Xia, H., Wan, Y., Yan, F., & Lu, L. (2014). Manganese oxide thin films prepared by pulsed laser deposition for thin film microbatteries. Materials Chemistry and Physics, 143(2), 720-727. [17] Zayed, M. F., & Eisa, W. H. (2014). Phoenix dactylifera L. leaf extract phytosynthesized gold nanoparticles; controlled synthesis and catalytic activity. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 121, 238-244. [18] Zayed, M. F., Eisa, W. H., & Shabaka, A. A. (2012). Malva parviflora extract assisted green synthesis of silver nanoparticles. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 98, 423-428.
Copyright
Copyright © 2024 Anil Kumar, Dr. Anil Kumar Shrotriya. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET63874
Publish Date : 2024-08-03
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

